Galvanization is widely used and cost-effective corrosion protection methods in manufacturing. From large-scale infrastructure and automotive production to CNC-machined components and sheet metal parts, galvanization plays a critical role in extending service life, reducing maintenance costs, and improving long-term reliability.
As global demand for durable steel components continues to grow, understanding how galvanization works—and which method is best for your application—has become essential for engineers, designers, and procurement teams alike.
This guide provides a comprehensive, engineering-level explanation of galvanization, covering its principles, processes, methods, advantages, limitations, and real-world applications, while helping you make informed material and surface-treatment decisions.
What Is Galvanization?

Galvanization is a surface treatment process that applies a protective zinc coating to steel or iron to prevent corrosion. Zinc serves two critical functions:
Barrier Protection – It physically isolates the base metal from moisture, oxygen, and corrosive chemicals.
Sacrificial Protection – Zinc corrodes preferentially, protecting the steel even if the coating is damaged.
Unlike paint or organic coatings, galvanization forms a metallurgical bond with the base metal, making it significantly more durable and long-lasting under mechanical stress and outdoor exposure.
Although steel is the most commonly galvanized material, iron and certain iron-based alloys can also be treated using zinc-based processes.
A Brief History of Galvanization
The concept of galvanization dates back to the 18th century and is named after Luigi Galvani, whose work on electrochemical reactions laid the foundation for understanding galvanic corrosion.
Key historical milestones include:
1836: Stanislas Sorel patents the modern hot-dip galvanizing process in France
1837: William Crawford patents the process in the UK
Mid-19th century: Rapid adoption in roofing, infrastructure, and industrial manufacturing
By 1850, zinc consumption for galvanization exceeded 10,000 tons annually, marking the beginning of large-scale industrial use. Today, galvanization remains a cornerstone technology across construction, automotive, energy, and manufacturing sectors.
How Does Galvanization Work?
At its core, galvanization relies on electrochemical principles. When steel is coated with zinc, the zinc acts as an anode, while the steel becomes the cathode.
When exposed to moisture or electrolytes:
Zinc oxidizes first
Steel remains protected
A stable zinc carbonate patina forms over time, further slowing corrosion
This means galvanized steel continues to resist rust even when scratched or cut, a key advantage over paint-based coatings.
Chemical Principles Behind Galvanization
Galvanization exploits galvanic corrosion, where two dissimilar metals are electrically connected in a corrosive environment.
Zinc has a lower electrode potential than iron
Zinc sacrifices itself to protect steel
Corrosion products form a dense, insoluble protective layer
This self-healing behavior is what makes galvanization especially valuable in outdoor, marine, and industrial environments.
Steps of the Galvanization Process
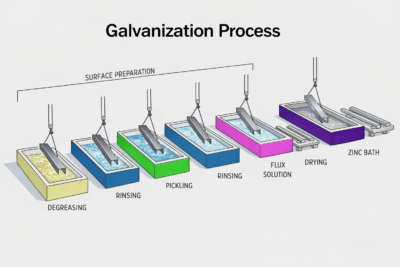
A high-quality galvanized coating depends on precise process control across multiple stages:
Degreasing & Cleaning – Removes oils, dirt, and organic contaminants
Pickling – Acid bath removes rust and mill scale
Rinsing – Prevents acid carryover
Fluxing – Prevents oxidation and improves zinc adhesion
Zinc Coating – Metal is coated using the selected galvanization method
Cooling or Quenching – Solidifies the coating
Inspection & Testing – Thickness, adhesion, and uniformity verification
Proper surface preparation is non-negotiable—galvanizing over rust or contamination is ineffective.
Benefits of Galvanization
Galvanization offers a unique balance of performance, cost efficiency, and durability:
Exceptional corrosion resistance
Long service life (25–50+ years in many environments)
Lower lifecycle cost than stainless steel
Minimal maintenance
Excellent impact and abrasion resistance
Full coverage on edges and internal surfaces
Environmentally sustainable and recyclable
For many applications, galvanized steel delivers the best return on investment when long-term durability matters.
How Galvanization Prevents Rust
Galvanization protects steel through:
Barrier protection – blocks corrosive agents
Sacrificial action – zinc corrodes first
Patina formation – zinc carbonate slows further oxidation
Even damaged coatings continue to protect exposed steel—an advantage unmatched by most surface finishes.
Types of Galvanization Methods
Different applications require different galvanization approaches.
Hot-Dip Galvanizing
Steel is immersed in molten zinc (~450 °C), forming a thick, metallurgically bonded coating.
Pros
Thick, durable coating
Cost-effective for large parts
Excellent outdoor performance
Cons
Variable thickness
Not ideal for tight tolerances
Pre-Galvanizing (Mill Galvanizing)
Continuous sheet or coil processing before fabrication.
Best for: Sheet metal, framing, tubing
Limitation: Cut edges require secondary protection
Electrogalvanizing
Zinc applied via electrochemical deposition.
Pros
Smooth, uniform appearance
Precise thickness control
Cons
Thinner coating
Lower corrosion resistance than hot-dip
Sherardizing / Thermal Diffusion Galvanizing
Zinc diffuses into steel at elevated temperatures using zinc powder.
Ideal for
Fasteners
Threads
Complex geometries
Galvannealing
Hot-dip galvanizing followed by annealing to form a zinc-iron alloy.
Advantages
Superior paint adhesion
Excellent weldability
Common in: Automotive manufacturing
Mechanical Plating
Cold process using tumbling and impact bonding.
Best for
Small, heat-sensitive parts
Uniform coating on intricate shapes
Continuous Galvanizing
High-speed zinc coating of steel strips or wires in production lines.
Advantages
High throughput
Consistent quality
Tools and Equipment Used in Galvanization
Zinc baths and kettles
Pickling and flux tanks
Conveyors and cranes
Thickness gauges
Temperature sensors
Ventilation and fume extraction
PPE and safety systems
Key Galvanization Parameters
Critical process variables include:
Zinc bath temperature
Immersion time
Zinc purity
Steel chemistry
Flux concentration
Withdrawal speed
Cooling rate
Environmental conditions
Process control directly determines coating quality and lifespan.
Materials Suitable for Galvanization
Carbon steel
Structural steel
Iron and iron-based alloys
Special consideration is required for high-strength steels due to hydrogen embrittlement risks.
Common Applications of Galvanization
Galvanized materials are used across nearly every major industry:
Construction and structural steel
Infrastructure and utilities
Automotive body panels
Agricultural equipment
Plumbing systems
Industrial machinery
Marine and coastal installations
Electronics and appliances
Galvanization integrates seamlessly with CNC machining, sheet metal fabrication, and welding workflows.
Disadvantages and Limitations
Despite its advantages, galvanization has limitations:
Hydrogen embrittlement risks
Aesthetic constraints
White rust formation
Thickness variation
Post-processing exposure
Understanding these helps ensure correct method selection.
How Long Does Galvanization Take?
Preparation: Several hours
Zinc immersion: Seconds to minutes
Cooling & inspection: Additional time
Continuous lines operate far faster than batch processing.
How Long Does Galvanized Steel Last?
Typical service life:
25–50 years in most environments
50–75 years in rural conditions
Shorter lifespan in marine or industrial settings
Thickness, environment, and maintenance strongly influence longevity.
Galvanized Steel vs Stainless Steel
| Factor | Galvanized Steel | Stainless Steel |
| Cost | Lower | Higher |
| Corrosion Resistance | Moderate–High | Very High |
| Maintenance | Low | Very Low |
| Applications | Structural, outdoor | Medical, marine |
Galvanized steel is often the smarter choice when cost and durability must be balanced.
How to Identify Galvanized Steel
Visible zinc spangle
Gray or matte finish over time
White corrosion products instead of red rust
Reduced spark intensity when ground
Chemical & Physical Properties of Galvanized Steel
Strong atmospheric corrosion resistance
Retains base metal strength
Magnetic
Suitable for moderate thermal exposure
Conclusion
Galvanization remains one of the most reliable and economical methods for protecting steel against corrosion. By selecting the correct galvanization process, manufacturers can dramatically extend product life, reduce maintenance costs, and improve long-term performance.
Whether you are designing structural components, sourcing CNC-machined parts, or selecting surface treatments for industrial equipment, galvanization offers a proven solution that balances performance and cost.
FAQs
- Can galvanized steel rust over time?
Yes, but far slower than untreated steel. Zinc protection significantly delays corrosion. - Can galvanized steel be painted?
Yes, with proper surface preparation and compatible primers. - Is galvanized steel weldable?
Yes, but zinc fumes require ventilation, and post-weld protection is recommended. - Can you galvanize rusty steel?
No. Rust must be completely removed before galvanization. - Is galvanization environmentally friendly?
Yes. Zinc is recyclable, and galvanized steel has a long service life.
Looking for Galvanized or Corrosion-Resistant Metal Parts?
If you need custom CNC machining, sheet metal fabrication, or surface treatment solutions, our engineering team can help you select the optimal material and galvanization method for your application.
Contact us today for a fast technical consultation and quotation.

